Our Products News
-

 Ribo Announces siRNA-based Treatment of Autoimmune Diseases (RBD2080) Received Clinical Approval in AustraliaRibo Announces siRNA-based Treatment of Autoimmune Diseases (RBD2080) Received Clinical Approval in AustraliaFebruary 26, 2025
Ribo Announces siRNA-based Treatment of Autoimmune Diseases (RBD2080) Received Clinical Approval in AustraliaRibo Announces siRNA-based Treatment of Autoimmune Diseases (RBD2080) Received Clinical Approval in AustraliaFebruary 26, 2025 -

 First Milestone Successfully Achieved in Ribo’s Collaboration in Liver Diseases With Boehringer IngelheimFirst Milestone Successfully Achieved in Ribo’s Collaboration in Liver Diseases With Boehringer IngelheimJanuary 10, 2025
First Milestone Successfully Achieved in Ribo’s Collaboration in Liver Diseases With Boehringer IngelheimFirst Milestone Successfully Achieved in Ribo’s Collaboration in Liver Diseases With Boehringer IngelheimJanuary 10, 2025 -

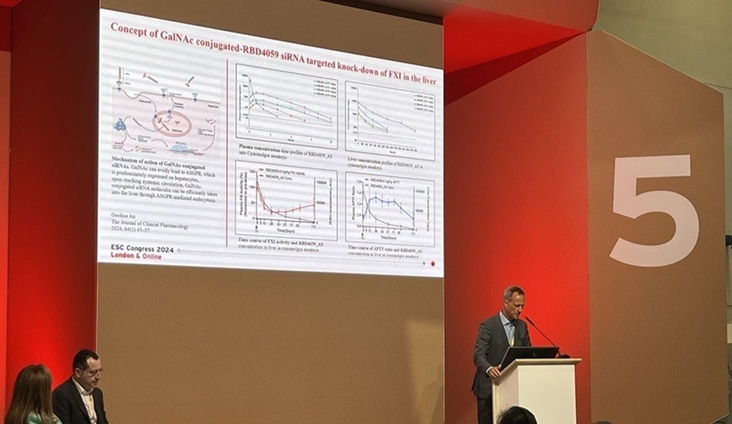 Ribo Presents First-In-Class FXI Targeting siRNA Drug RBD4059 Phase I Clinical Data At ESC Congress 2024Ribo Presents First-In-Class FXI Targeting siRNA Drug RBD4059 Phase I Clinical Data At ESC Congress 2024August 31, 2024
Ribo Presents First-In-Class FXI Targeting siRNA Drug RBD4059 Phase I Clinical Data At ESC Congress 2024Ribo Presents First-In-Class FXI Targeting siRNA Drug RBD4059 Phase I Clinical Data At ESC Congress 2024August 31, 2024 -

 Innovating for a Healthier Tomorrow: Ribo Role in Transforming Liver Disease Treatment with siRNAInnovating for a Healthier Tomorrow: Ribo Role in Transforming Liver Disease Treatment with siRNA.April 8, 2024
Innovating for a Healthier Tomorrow: Ribo Role in Transforming Liver Disease Treatment with siRNAInnovating for a Healthier Tomorrow: Ribo Role in Transforming Liver Disease Treatment with siRNA.April 8, 2024 -

 Boehringer Ingelheim partners with Ribo to develop new treatments for people with liver diseasesBoehringer Ingelheim partners with Ribo to develop new treatments for people with liver diseasesJanuary 3, 2024
Boehringer Ingelheim partners with Ribo to develop new treatments for people with liver diseasesBoehringer Ingelheim partners with Ribo to develop new treatments for people with liver diseasesJanuary 3, 2024 -

 Ribo and Qilu Announce Licensing Agreement for siRNA Therapeutic Targeting PCSK9Ribo and Qilu Announce Licensing Agreement for siRNA Therapeutic Targeting PCSK9December 25, 2023
Ribo and Qilu Announce Licensing Agreement for siRNA Therapeutic Targeting PCSK9Ribo and Qilu Announce Licensing Agreement for siRNA Therapeutic Targeting PCSK9December 25, 2023




